A) solid, liquid, and gas are all in equilibrium.
B) the vapor pressure of the solid is equal to the vapor pressure of the liquid.
C) the rate of sublimation is equal to the rate of evaporation.
D) a & b
E) all of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which do you predict will have the largest intermolecular attraction F2, Cl2, Br2, I2?
A) F2
B) Cl2
C) Br2
D) I2
E) they will all be the same
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which do you think will have the highest surface tension?
A) water
B) liquid N2
C) CCl4
D) CHCl3
E) molten NaCl
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A supercritical fluid has a density that
A) is lower than a liquid.
B) is lower than a gas.
C) is higher than a liquid.
D) is higher than a gas.
E) can be varied from gas-like to liquid-like.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which do you think has a higher boiling point, n-butanol or tert-butanol? 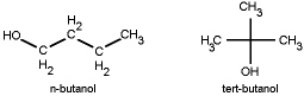
A) n-butanol because it forms stronger hydrogen bonds.
B) tert-butanol because it forms stronger hydrogen bonds.
C) n-butanol because the linear molecule allows for greater dispersion forces.
D) tert-butanol because the tetrahedral molecule allows for greater dispersion forces.
E) they will be exactly the same.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The triple point of a compound is at 38.2°C and a pressure of 0.75 atm. A gaseous sample of the substance is held at a constant pressure of 0.79 atm. Initially the sample is at 50°C and is slowly cooled until it is -25°C. What if any phase transitions occur?
A) condensation
B) deposition
C) condensation followed by freezing
D) simultaneous condensation and freezing
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What types of intermolecular forces would you expect to find in CHCl3?
A) dispersion
B) dipole-dipole
C) hydrogen bonding
D) a & b
E) all of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which has the highest vapor pressure at room temperature?
A) methanol
B) ethanol
C) propanol
D) butanol
E) there is no way to predict
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Generally if a liquid has stronger intermolecular attractions it will have
A) a higher viscosity.
B) a higher vapor pressure.
C) a higher boiling point.
D) a & b
E) b & c
F) a & c
G) all of the above
I) E) and F)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 20°C water has a vapor pressure of 0.0231 atm. If you bubble air through a container of water and collect 1 L of it at a total pressure of 1 atm, how many grams of water are in your sample of air (you can assume it is saturated) ?
A) 9.6×10-4
B) 3.0×10-4
C) 3.0×10-3
D) 1.1×10-2
E) 1.7×10-2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Dipole-dipole forces are
A) larger than ion-ion forces.
B) about the same as all ion-ion forces.
C) much smaller than ion-ion forces.
D) between the force for °1 ions and °2 ions.
E) about a factor of two smaller the attraction of °1 ions.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The process of a solid converting directly to a gas is called
A) evaporation
B) condensation
C) deposition
D) sublimation
E) transformation
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the phase diagram of water increasing pressure leads to lower melting temperatures.
A) this is true for all compounds
B) this is true for all polar compounds
C) this is true for all hydrogen bonding compounds
D) this is true for compounds with solid phases that are less dense than their liquid phases
E) this is true only for H2O, HF, and NH3
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 33 of 33
Related Exams