A) N
B) C
C) O
D) P
E) Al
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has no net dipole moment?
A) N2O
B) NF3
C) H2Se
D) TeO3
E) CH3Cl
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
In order for a noncyclic triatomic molecule to be bent, VSEPR theory requires that there must be two lone pairs on the central atom.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following structures does the central atom have a formal charge of +2?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules does not have a dipole moment?
A) CS2
B) H2S
C) CH2Cl2
D) PH3
E) CH2O
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the Lewis structure in which formal charges are minimized for the periodate anion, IO4-.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Boron never achieves an octet in any of its compounds.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements would you expect most likely to be a central atom with an expanded valence shell?
A) Ne
B) P
C) N
D) C
E) B
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following Lewis structures is definitely incorrect?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
A molecule that contains polar bonds will always have a dipole moment.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of NO2- as predicted by the VSEPR theory?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) resonant
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX4 will have a ______ molecular shape.
A) bent
B) trigonal planar
C) trigonal pyramidal
D) square planar
E) tetrahedral
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of BCl3 as predicted by the VSEPR theory?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the actual bond angle in SeCl2 using the VSEPR theory.
A) more than 120°
B) between 109° and 120°
C) between 90° and 109°
D) exactly 90°
E) less than 90°
G) B) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the molecular shape of ClO3F as predicted by the VSEPR theory? 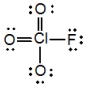
A) trigonal pyramidal
B) square planar
C) square pyramidal
D) tetrahedral
E) octahedral
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrazine, N2H4, is a good reducing agent that has been used as a component in rocket fuels. Select its Lewis structure.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
G) None of the above
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles around carbon in C2I2 using the molecular shape given by the VSEPR theory.
A) 90°
B) 109°
C) 120°
D) 180°
E) None of these choices are correct.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of HOF as predicted by the VSEPR theory?
A) trigonal pyramidal
B) trigonal
C) tetrahedral
D) linear
E) bent
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Thionyl chloride is used as an oxidizing and chlorinating agent in organic chemistry. Select the best Lewis structure for SOCl2.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
G) B) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX3E2 will have a _____ molecular shape.
A) trigonal pyramidal
B) trigonal bipyramidal
C) trigonal planar
D) T-shaped
E) see-saw
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 98
Related Exams